Unit 1: Atoms & Ions
Exploring the fundamental building blocks of matter and the charged particles they form.
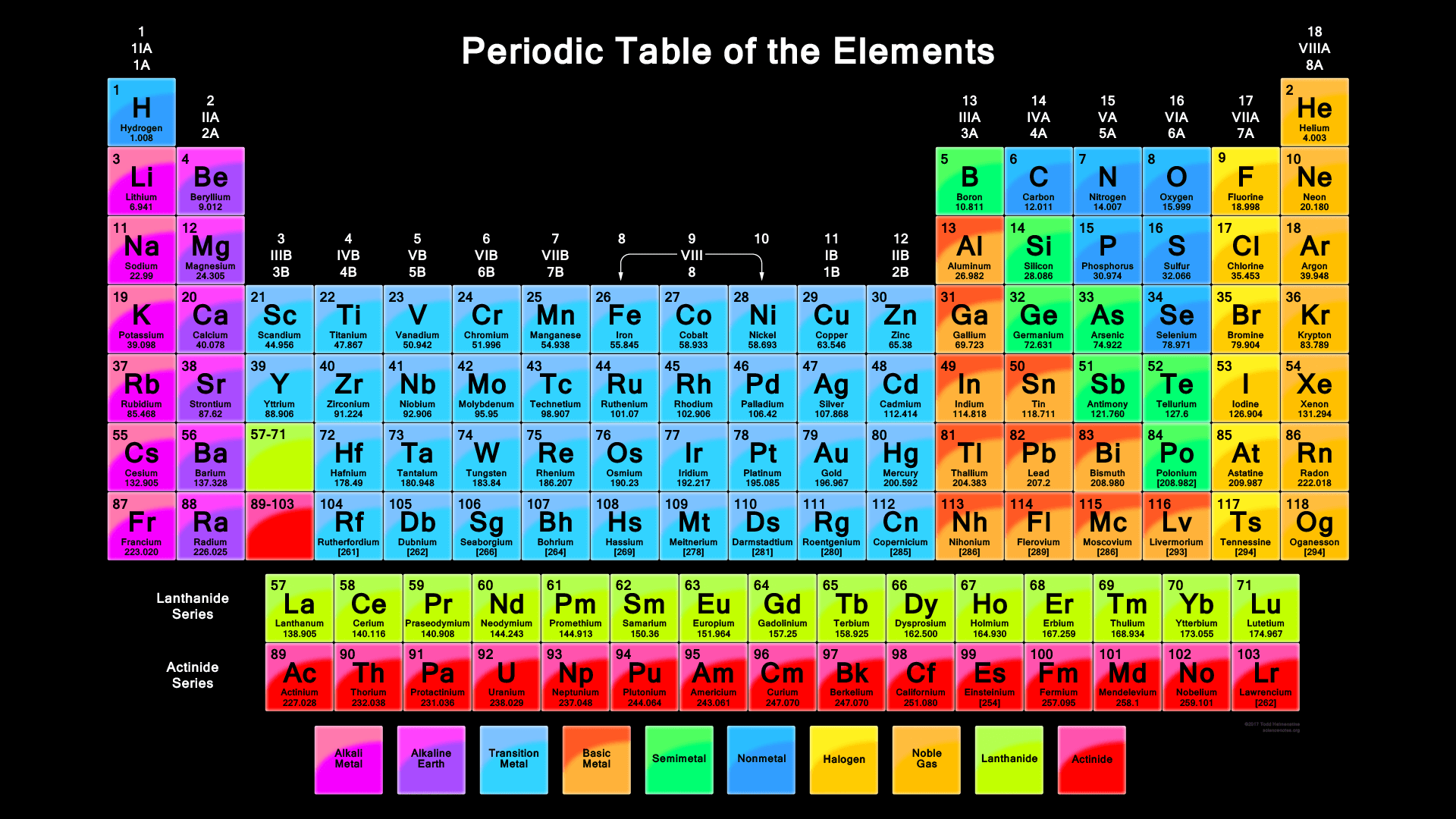
1.1 Names & Symbols ⚛️
Every element has a distinct one- or two-letter symbol that serves as a universal identifier. This modern system, largely credited to Jöns Jacob Berzelius in the 19th century, replaced older, more complex alchemical symbols. These symbols, standardized by the International Union of Pure and Applied Chemistry (IUPAC), are crucial for writing chemical formulas and equations. While many symbols are derived from the element's modern name (e.g., C for Carbon), others originate from older Latin or German names to avoid confusion with other elements.
Solved Examples:
- What is the element and symbol for an atom with 11
protons?
Solution: The atomic number, $Z$, is 11. On the periodic table, the element with $Z=11$ is Sodium. Its symbol is Na, derived from its Latin name, *natrium*. - Explain why the symbol for gold is Au.
Solution: The symbol Au comes from the Latin word *aurum*, which means "shining dawn." This historical convention prevents confusion with other elements. - Find the name for the element with the symbol Ag.
Solution: The symbol Ag comes from the Latin word *argentum*. Therefore, the element is Silver. - Why is the symbol for Potassium 'K'?
Solution: The symbol K is derived from Potassium's Latin name, *kalium*. This was necessary because 'P' was already used for Phosphorus. - The element Tungsten has the symbol 'W'. Explain this
origin.
Solution: The symbol W comes from Tungsten's German name, *Wolfram*. This name originates from the mineral wolframite, from which it was first isolated.
1.2 Mass, Charges & Isotopes 🔬
An atom is composed of a central nucleus surrounded by electrons orbiting in specific paths known as shells or energy levels. Each shell can only hold a specific maximum number of electrons. The three main subatomic particles are:
- Protons (p⁺): Located in the nucleus, with a positive charge (+1) and a relative mass of ~1 amu.
- Neutrons (n⁰): Also in the nucleus, with no charge and a relative mass of ~1 amu.
- Electrons (e⁻): Orbit the nucleus, with a negative charge (-1) and a negligible relative mass (~1/1836 amu).
| Particle | Relative Charge | Relative Mass (amu) |
|---|---|---|
| Proton | +1 | ~1 |
| Neutron | 0 | ~1 |
| Electron | -1 | ~1/1836 |
Mass Number and Isotopes
An element is represented using the notation: $$ _{Z}^{A}X $$ Where $X$ is the element symbol, $A$ is the mass number, and $Z$ is the atomic number.
For example, Chlorine has two main isotopes: Chlorine-35 ($_{17}^{35}$Cl) and Chlorine-37 ($_{17}^{37}$Cl). Both have 17 protons, but they have 18 and 20 neutrons, respectively. Because they have the same number of electrons, isotopes of an element have identical chemical properties.
Ions
- A cation is a positively charged ion, formed when a metal atom loses electrons (e.g., Mg → Mg²⁺ + 2e⁻).
- An anion is a negatively charged ion, formed when a non-metal atom gains electrons (e.g., O + 2e⁻ → O²⁻).
Solved Examples:
- Determine the number of protons, neutrons, and electrons in a neutral atom of
$_{82}^{207}$Pb.
Solution:
- Protons = $Z$ = 82.
- Electrons = Protons (since it's neutral) = 82.
- Neutrons = $A - Z = 207 - 82$ = 125. - Calculate the subatomic particles in an oxide ion,
$_{8}^{16}$O²⁻.
Solution:
- Protons = $Z$ = 8.
- Neutrons = $A - Z = 16 - 8$ = 8.
- The 2- charge means it has gained 2 electrons. A neutral atom has 8 electrons, so the ion has $8 + 2 = 10$ electrons. - An ion has 12 protons, 12 neutrons, and 10 electrons. Write its complete
symbol.
Solution:
- 12 protons means the element is Magnesium (Mg) and $Z=12$.
- Mass number $A = p^+ + n^0 = 12 + 12 = 24$.
- Charge = (protons) - (electrons) = $12 - 10 = +2$.
- The complete symbol is $_{12}^{24}$Mg²⁺. - Compare the isotopes of Hydrogen: Protium ($_{1}^{1}$H), Deuterium ($_{1}^{2}$H), and
Tritium ($_{1}^{3}$H).
Solution: All three are isotopes of Hydrogen because they each have 1 proton. They differ in their number of neutrons: Protium has 0, Deuterium has 1, and Tritium has 2. This gives them different masses but identical chemical properties. - An ion X³⁺ has a mass number of 56 and contains 30 neutrons. Identify element
X.
Solution:
- First, find the number of protons ($Z$): $Z = A - N = 56 - 30 = 26$.
- The element with 26 protons is Iron (Fe). The element is Iron.
1.3 Relative Atomic Mass ⚖️
Think of it like calculating your final grade. Your exam might be worth 60% of the grade, while homework is worth 40%. The relative atomic mass calculation is similar: it "weighs" the mass of each isotope by its natural abundance to find the overall average mass. This is why the values on the periodic table are not whole numbers.
$$ A_r = \frac{\sum (\text{abundance} \% \times \text{isotopic mass})}{100} $$Solved Examples:
- Chlorine exists as two isotopes: $^{35}$Cl (75.77% abundance, mass 34.97 amu) and $^{37}$Cl
(24.23% abundance, mass 36.97 amu). Calculate its relative atomic
mass.
Solution:
$A_r = \frac{(75.77 \times 34.97) + (24.23 \times 36.97)}{100}$
$A_r = \frac{2649.6 + 895.8}{100} = \frac{3545.4}{100} = 35.45 \ \text{amu}$ - Lithium has two stable isotopes: $^{6}$Li (7.5% abundance) and $^{7}$Li (92.5% abundance).
Calculate the relative atomic mass of lithium.
Solution:
$A_r = \frac{(7.5 \times 6.0) + (92.5 \times 7.0)}{100}$
$A_r = \frac{45 + 647.5}{100} = \frac{692.5}{100} = 6.925 \ \text{amu}$ - Magnesium has three isotopes: $^{24}$Mg (78.99%, mass 23.99 amu), $^{25}$Mg (10.00%, mass
24.99 amu), and $^{26}$Mg (11.01%, mass 25.98 amu). Calculate its relative atomic
mass.
Solution:
$A_r = \frac{(78.99 \times 23.99) + (10.00 \times 24.99) + (11.01 \times 25.98)}{100}$
$A_r = \frac{1895.0 + 249.9 + 286.0}{100} = \frac{2430.9}{100} = 24.309 \ \text{amu}$ - Boron has a relative atomic mass of 10.81 amu and consists of two isotopes, $^{10}$B (mass
10.01 amu) and $^{11}$B (mass 11.01 amu). Calculate the natural abundance of each
isotope.
Solution:
- Let the abundance of $^{10}$B be $x$. The abundance of $^{11}$B is $(100 - x)$.
- Set up the equation: $10.81 = \frac{(x \times 10.01) + ((100 - x) \times 11.01)}{100}$
- Solve: $1081 = 10.01x + 1101 - 11.01x \implies 1.00x = 20 \implies x = 20$.
- The abundance of $^{10}$B is 20% and $^{11}$B is 80%. - Does the formation of an Fe³⁺ ion significantly change its mass from a neutral Fe atom?
Explain.
Solution: No, the mass change is negligible. An Fe³⁺ ion is formed by losing three electrons. Since the mass of an electron (~1/1836 amu) is extremely small compared to a proton or neutron, the loss of a few electrons does not have a measurable impact on the atom's overall mass for most practical calculations.
Featured Tutorial Videos
Credit: Tidlybit
Credit: Khan Academy